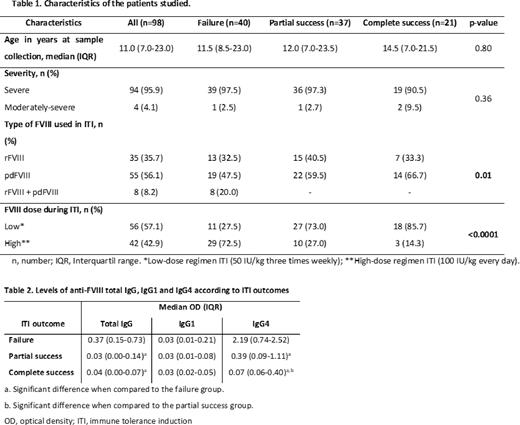
Background: The development of inhibitors in patients with inherited hemophilia A (PwHA) is an important complication of the treatment with exogenous factor VIII (FVIII), leading to inefficient replacement therapy. Immune tolerance induction (ITI) is the standard treatment to eradicate inhibitors in HA. The immune biology and biomarkers related with ITI outcome are still unclear. Few studies have investigated this, of which most included few PwHA. Furthermore, such studies have not assessed samples in specific time-points, but have rather grouped patients into categories of inhibitors and non-inhibitors. Aims: This study aimed to investigate specific anti-FVIII immunoglobulins IgG1, IgG4 and total IgG as biomarkers of ITI outcome at the completion of ITI. Methods: We included PwHA from 15 hemophilia treatment centers, participants of the Brazilian Immune Tolerance (BrazIT) Cohort Study. Inclusion criteria were severe and moderately-severe (<2% FVIII) PwHA, with high-responding (>5.0BU/mL) inhibitors, who completed a first course of ITI. All patients were treated with the same ITI protocol, i.e., 50 international units (IU)/kilogram (kg) of FVIII. In case ITI titers did not decrease by at least 20% first 3-6 months after inhibitor peak, FVIII was increased to 100 IU/kg daily. We collected socio-demographic, clinical and laboratory data using standardized forms. Outcome of ITI was defined according to published classification as failure, partial and total successes. For this analysis, plasma samples were collected in citrate at completion of ITI and frozen at -80°C until assessment. We measured plasma levels of anti-FVIII IgG1, IgG4 and total IgG in ELISA using immobilized recombinant (r) FVIII (alfaoctocogue, Takeda, USA). Local ethics committees approved this study and all patients/guardians signed informed consent. Results: We included 98 PwHA, median age of 11.0 years (interquartile range [IQR] 7.0-23.0), 94 (95.9%) severe HA (<1%). According to ITI outcome, 40 (40.8%) had ITI failure, 37 (37.8%) had partial success and 21 (21.4%) had total success (Table 1). Levels of total IgG in patients who failed ITI (median optical density [OD], 0.37; IQR 0.15-0.73) were significantly higher when compared to the levels of patients with partial (median OD, 0.03; IQR 0.00-0.14; p<0.0001) and complete (median OD, 0.04; IQR 0.00-0.07; p<0.0001) success. Levels of total IgG anti-FVIII were not different between partial and complete success groups. Levels of anti-FVIII IgG4 were significantly higher in PwHA who failed ITI (median OD, 2.19; IQR 0.74-2.52) compared with those who had partial (median OD, 0.39; IQR 0.09-1.11; p<0.0001) and complete success (median OD, 0.07; IQR 0.06-0.40; p<0.0001). Anti-FVIII IgG4 levels were higher in PwHA presenting partial success compared with those with complete success (p=0.02). Levels of anti-FVIII IgG1 were not different between PwHA who failed ITI (median OD, 0.03; IQR 0.01-0.21), in comparison with the ones who achieved partial success (median OD, 0.03; IQR 0.01-0.08; p=0.35) and achieved complete success (median OD, 0.03; IQR 0.02-0.05; p=0.72) (Table 2). Conclusion: We conclude that anti-FVIII IgG4 and IgG total are good biomarkers of ITI outcome. Levels of IgG4 are associated with ITI outcome in a dose-dependent manner, with failure>partial>complete success. Anti-FVIII IgG1 is not a useful biomarker of ITI outcome. This study was fully supported by Brazilian Governmental grants from Fapemig, CNPq and Ministry of Health of Brazil (Fundo Nacional de Saúde).
Disclosures
Camelo:Roche and Takeda: Honoraria, Other: Scientific event grants, Speakers Bureau. Oliveira:NovoNordisk: Other: Scientific event grants, Speakers Bureau; Takeda: Other: Scientific event grants, Speakers Bureau. Callado:Hoffman-La Roche: Other: Scientific event grants; NovoNordisk: Other: Scientific event grants. Cerqueira:Hoffman-La Roche: Other: Scientific event grants; NovoNordisk: Other: Scientific event grants. Etto:Hoffman-La Roche: Other: Scientific event grants. Roberti:Hoffman-La Roche: Other: Scientific event grants; Takeda: Other: Scientific event grants; Brazilian Ministry of Health: Consultancy; NovoNordisk: Other: Scientific event grants, Speakers Bureau. Anegawa:Hoffman-La Roche: Other: Scientific event grants; Takeda: Other: Scientific event grants. Pinto:Roche: Honoraria, Other: Scientific event grants, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal